ESPOIR trial 5 year-data
Early results from the prospective ESPOIR-Trial have indicated excellent results for pulmonary valve replacement (PVR) using decellularized pulmonary homografts (DPH). In 2021, a 5-year analysis of ESPOIR-Trial patients was performed to provide an insight into the midterm DPH performance. ESPOIR Trial and Registry patients were matched with patients having received bovine jugular vein conduits (BJV) and cryopreserved homografts (CH) considering patient age, type of heart defect, and previous procedures to present the overall experience with DPH.
121 patients (59 female) had been prospectively enrolled between 8/2014 and 12/2016 with a mean age of 21.3 years (SD 14.4), DPH diameter 24.4 mm (2.8). One death (73-year-old) occurred during a mean follow-up of 5.3 years (0.9), in addition to 2 perioperative deaths resulting in an overall mortality-rate of 2.5 %. One case of endocarditis in 637 patient years was noticed, resulting in an incidence of 0.15 % per patient-year. At 5 years, the mean peak gradient was 19.9 mmHg (9.9), the mean regurgitation 0.9 (0.6, Grade 0-3) and the freedom from explantation/any reintervention was 97.5 % (1.5).
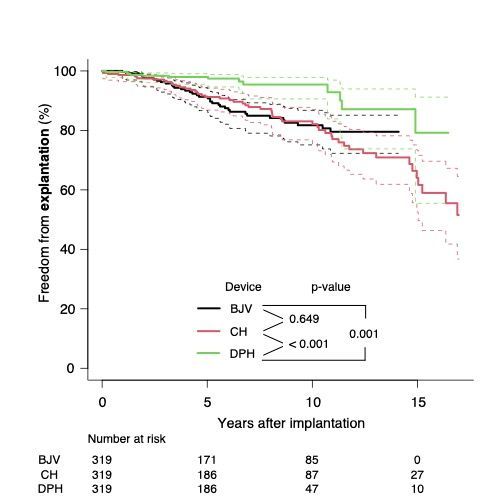
The combined DPH cohort, n=319, comprising both Trial and Registry data, showed significantly better freedom from explantation, DPH 95.5 % (SD 1.7), CH 83.0 % (2.8), p<0.001, and BJV 81.7 % (2.9), p=0.001, and less structural valve degeneration at 10 years when matched to CH, n=319, and BJV, n=319, DPH 65.5 % (SD 4.4), CH 47.3 % (3.7), p=0.11, BJV 39.6 % (3.9), p<0.001.
In conclusion, 5-year data of the prospective ESPOIR-Trial showed excellent performance for DPH and low rates of adverse events. ESPOIR Registry data up to 15 years, including a matched comparison with CH and BJV, demonstrated statistically significant better freedom from explantation or functional explantation.